In the past, some compounding pharmacies have had issues with the quality of the product they’re distributing, resulting in harsher restrictions from the Federal Drug Administration. In order to take control of the quality assurance from compounding pharmacies, the FDA divided these pharmacies into two designations: 503A and 503B. In this article, we’ll explore 503B compounding pharmacies, their benefits and what makes them different from 503A.
What is a Compounding Pharmacy?
Before delving into the differences between the two pharmacy designations, it’s important to understand the role of compounding pharmacies. They’re not exactly drug manufacturers and not just drug distributors—they’re hybrid facilities that can do both. Traditionally, compounding pharmacies existed to make drugs for specific patients with individual needs that couldn’t be met by widely used commercial drugs.
For example, one patient may be allergic to the ingredients in a commercially produced version of a drug, so a compounding pharmacy’s role would be to recreate the drug without the ingredient to which the patient is allergic.

However, the role of compounding pharmacies has since expanded. Particularly during drug shortages, compounding pharmacies will step in at the local level to reduce the shortage’s impact on patients. Recently, drug shortages have been trending for longer periods of time and for more important drugs than before. For that reason, compounding pharmacies have become more important than ever.
Who Regulates Compounding Pharmacies?
The FDA regulates drug manufacturers and individual state governments regulate pharmacies. Compounding pharmacies are neither and both at once, so who regulates them? The answer is actually three different government agencies: the individual state Boards of Pharmacy, the FDA and the Drug Enforcement Administration.
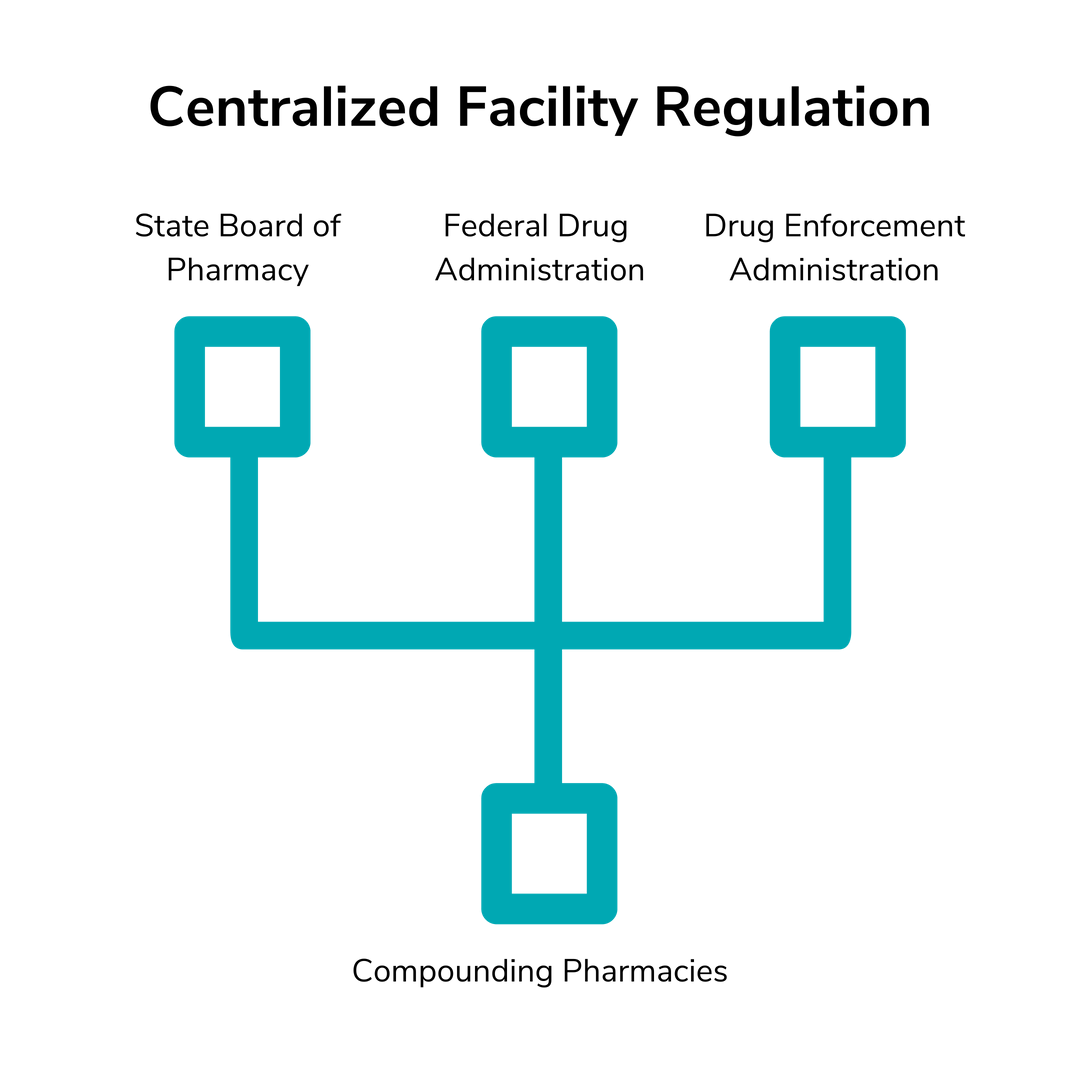
Each state’s Board of Pharmacy investigates and reports on compounding pharmacies to ensure that they follow the state’s regulations of pharmacy practice. The FDA monitors the integrity and quality of the drugs, specifically regarding the active ingredients. The DEA monitors any handling of controlled substances. So, while the perception may be that compounding pharmacies aren’t regulated enough, they’re actually under even more scrutiny and regulation than traditional manufacturers and pharmacies.
Comparing 503A vs. 503B Designations
The comparison chart below highlights the major differences between the 503A and 503B designations of compounding pharmacies. These designations are specifically for the facilities preparing the compounded drugs, not the drugs themselves.
Compounding Standards & Requirements |
503A | 503B |
| Compliant with U.S. Pharmacopeial chapters 795 & 797 | ✅ | ✅ |
| Compliant with state Boards of Pharmacy | ✅ | ✅ |
| Produce medications for use in clinics, hospitals & surgery centers | ???? | ✅ |
| cGMP Compliant with CFR section 21-210 & 21-211 | ???? | ✅ |
| Human-sterile medications not required to be patient-specific | ???? | ✅ |
| Environmental monitoring performed per production shift in primary compounding areas (ISO 5) | ???? | ✅ |
| Environmental monitoring performed weekly in secondary compounding areas (ISO 7 & 8) | ???? | ✅ |
| Labeling requirements regulated by the Drug Quality and Security Act (DQSA) | ???? | ✅ |
| Autonomous quality assurance department required for product investigation | ???? | ✅ |
| Scientific testing required to confirm stability of medication when subjected to degradation (time, temperature & humidity) | ???? | ✅ |
| Required to register with each state Board of Pharmacy, DEA and FDA | ???? | ✅ |
| Required to report product list to FDA biannually | ???? | ✅ |
In summary, both the 503A and 503B designations play important roles in the healthcare industry, just at different levels. Neither is more important than the other. 503B pharmacies are held to stricter regulations on the process of production than 503A pharmacies, but can also produce on a grander scale.
Understanding the Role of 503A Pharmacies
The 503A designation is classified as a compounding pharmacy that prepares medications at a microeconomic level, only within the United States. 503A pharmacies have different regulations and are designed for specialty compounded patient medications that are produced in smaller batches and distributed to individuals. Learn more about our 503A compounding facility today.
Understanding the Role of 503B Pharmacies
The 503B pharmacy designation is classified as a compounding pharmacy that prepares medications at a large-scale, mass-production level. Another commonly used term for a 503B pharmacy is an “FDA outsourcing facility.” These large-batch manufacturing facilities are empowered to reduce the per-unit cost of medications. Also, 503B pharmacies are the only type of compounding pharmacy authorized to produce medications for office use.

The Benefits of 503B Pharmacies
While both 503A and 503B designations of compound pharmacies are critical in their respective roles, there are many advantages of 503B pharmacies that are worth mentioning:
- Quality Assurance: 503B pharmacies operate under cGMP guidelines, the same guidelines as pharmaceutical manufacturers. A better manufacturing environment makes for a better end product, and the regulations governing 503B pharmacies can guarantee they’re producing at a high quality.
- Market Outreach: 503A pharmacies aren’t allowed to sell their products beyond the state level, but 503B pharmacies can reach markets across the country. This permits 503B pharmacies to achieve a greater market share.
- Greater Efficiency: Having centralized, top-down management of a compounding pharmacy encourages greater efficiency and resource sharing.
- Good for Office Use: 503B pharmacies are the only compounding pharmacies that are allowed to provide office-use medications. This includes medications administered in physician offices, clinics and hospitals.
- Drug-Shortage Assistance: During a drug shortage, 503B pharmacies may step in to help boost productivity and increase the availability of medications affected by the shortage.
See why it can be beneficial to get medications like TriMix injections through a 503B compounding pharmacy like Olympia Pharmaceuticals today.



